Difference between revisions of "SMT profiling with pmcstat and perf"
| (26 intermediate revisions by the same user not shown) | |||
| Line 14: | Line 14: | ||
=== Benchmark code === | === Benchmark code === | ||
| + | ==== Description of comparison code (cmp_mp) ==== | ||
The code being profiled and used for benchmarking is the genomic comparison code that the [https://mpjanson.org M. P. Janson Institute for Analytical Medicine] uses to look for molecular mimicry between pathogens and human tissue and hormones. This code uses a variety of techniques to compare nucleotide and amino acid sequences. There are both byte-by-byte and vector versions. The raw source code can be found at [[TBD]] | The code being profiled and used for benchmarking is the genomic comparison code that the [https://mpjanson.org M. P. Janson Institute for Analytical Medicine] uses to look for molecular mimicry between pathogens and human tissue and hormones. This code uses a variety of techniques to compare nucleotide and amino acid sequences. There are both byte-by-byte and vector versions. The raw source code can be found at [[TBD]] | ||
| − | Fundamentally the algorithm just compares characters in two sequences for matching. The goal is to match consecutive changing characters and record the string and location of the matches. Unlike strstr or similar functions, the string to be matched is not known in advance. The first character in one sequence is compared against the first character in the other sequence. If it matches, the second characters are compared. If they match, the third | + | Fundamentally the algorithm just compares characters in two sequences for matching. The goal is to match consecutive changing characters and record the string and location of the matches. Unlike strstr or similar functions, the string to be matched is not known in advance. The first character in one sequence is compared against the first character in the other sequence. If it matches, the second characters are compared. If they match, the third characters are compared, and so on, until the characters do not match. If the length of the matched string is below the minimum desired length, the match is discarded and the comparisons start over at the next character. This can "slide" the comparisons relative to each other, so if the first four characters match but not the fifth, the comparison starts over at the first character of one sequence and the fifth character of the other. If there is a match of sufficient length, that two-dimensional region is marked as an "exclusion region" and not searched again. |
| − | + | The code can run N number of user-specified pthreads in parallel (-j N), by slicing one of the two sequences into smaller pieces and stitching the results together. N must be an even number (one forward, one reverse). Some matches are lost at the boundaries between slices when one or both parts are less than the minimum length. The execution time is extremely sensitive to comparisons made; adding checks to determine if a match falls on a slice boundary adds significant overhead. For large sequences, the odds of a matching string falling on a boundary are very low; for small sequences, running fewer threads is effective without costing significant time delays. For absolute accuracy, run only two threads (one forward and one in reverse). | |
| − | The most common comparison method is BLAST, which uses a database of pre-identified nucleotide sequences. This is useful for rapidly finding large matches, but does not offer granularity for exploring other aspects of the genome. The above method allows matching of nucleotide and amino acid sequences on both position one and position two nucleotide. This often reveals "repeat motifs" where a sequence of nucleotides is repeated multiple times within a pathogen. The current thinking is that this is an "immuno-evasive strategy," allowing the pathogen to escape detection by the host's immune system. Additionally, by recording the location of the matches, the distance between matches can be calculated. The above algorithm also allows for detecting "SIDs," which are "Substitutions, Insertions, and Deletions," which occur regularly in rapidly evolving pathogens such as | + | ==== Description of data ==== |
| + | Nucleotides are denoted as A, T, C, and G. There are three nucleotides in an amino acid, and 23 amino acids in humans, which leads to "isocodon" amino acids. These have the same function but different nucleotide combinations. There are amino acids that denote the initiation and termination of a gene. The amino acid is determined by its offset from the initiation amino acid (ATG, methionine), which starts an "Open Reading Frame (ORF)." ORFs can overlap. DNA has a forward ("sense") and backward ("anti-sense") direction, so there are three possible amino acids reading forward, and three when reading backwards. Nucleotide sequences are usually published in the forward direction, and the other strand's nucleotide matching the forward direction is inferred (A matches T, and C matches G, and vice versa). Genome sequences can be obtained from the [https://www.ncbi.nlm.nih.gov/gene/ NIH National Library of Medicine] gene database, as well as in other places. The particular sequence is identified by its "accession number," and sequencing of the same organism by different labs does lead to different accession numbers. Some sequences are "canonical," meaning the generally accepted sequence that is often an assembly of accessions, and others are "contributions," meaning independently obtains sequences submitted for recording. | ||
| + | |||
| + | A typical "FASTA" file contains data as | ||
| + | |||
| + | <pre> | ||
| + | TATATTAGAGTAGGAGCTAGAAAATCAGCACCTTTAATTGAATTGTGCGTGGATGAGGCTGGTTCTAAATCACCCATTCAGTACATCGATATCGGTAATTATACAGTT... | ||
| + | </pre> | ||
| + | |||
| + | The most common comparison method is BLAST, which uses a database of pre-identified nucleotide sequences. This is useful for rapidly finding large matches, but does not offer granularity for exploring other aspects of the genome. The above method allows matching of nucleotide and amino acid sequences on both position one and position two nucleotide. This often reveals "repeat motifs" where a sequence of nucleotides is repeated multiple times within a pathogen. The current thinking is that this is an "immuno-evasive strategy," allowing the pathogen to escape detection by the host's immune system. Additionally, by recording the location of the matches, the distance between matches can be calculated. This is important in deciding if a host antibody would identify healthy tissue and hormones as pathogenic, by having similar shapes. The above algorithm also allows for detecting "SIDs," which are "Substitutions, Insertions, and Deletions," which occur regularly in rapidly evolving pathogens such as viruses. | ||
| + | |||
| + | ==== Sample output ==== | ||
| + | |||
| + | The output is a csv file that is run through some custom image generation code using libjpeg. An example of the processed data is below | ||
| + | |||
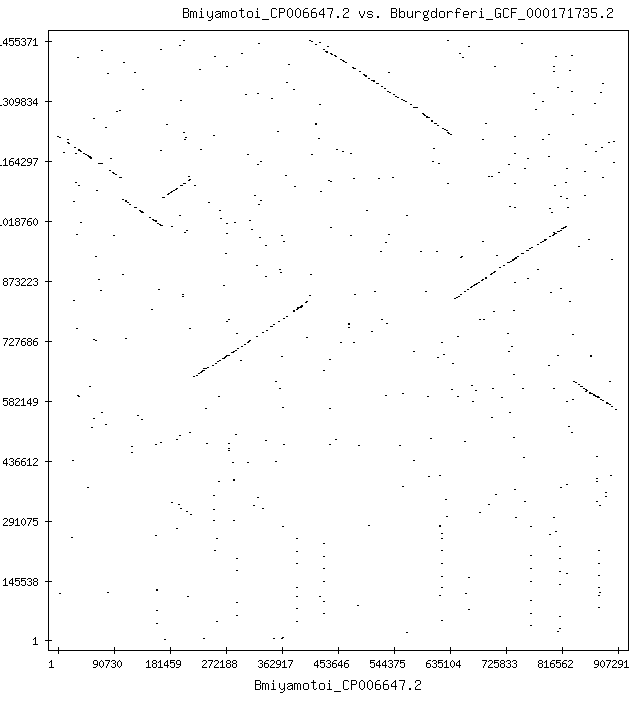
| + | <center>[[file:Mp_Bmiyamotoi_CP006647.2_vs_Bburgdorferi_GCF_000171735.2_20_1_100_nuc.gif]]</center> | ||
| + | |||
| + | The image shows matching sequences between two bacteria, [https://www.ncbi.nlm.nih.gov/nuccore?term=GCF_000171735.2 B. burgdorferi], which causes Lyme disease, and [https://www.ncbi.nlm.nih.gov/nuccore/CP006647.2/ B. miyamotoi], a bacteria in the same Borrelia family. Matches are occurring in both the forward and reverse directions. In this particular case, the B. miyamotoi sequence was assembled from fragments in both the "sense" and "antisense" direction. This is an artifact of how PCR is done, but has to be considered when doing analysis. Running only forward matching can lead to missed matches (a "false negative"). This comparison is particularly important because some people will exhibit symptoms of Lyme disease but only test weakly positive or not at all for Lyme disease. This is known in science but not practiced in medicine, leading to erroneous conclusions about the cause of the patient's symptoms (see [https://doi.org/10.1016/j.cmi.2019.07.026 Borrelia miyamotoi infection leads to cross-reactive antibodies to the C6 peptide in mice and men]). | ||
| + | |||
| + | The B. burgdefori sequence is 1,455,375 nucleotides long, and the B. miyamotoi sequence is 907,293 nucleotides long. Each nucleotide is compared at least once, and when there are partial matches too short to place the nucleotide into an exclusion region, it may be compared more than once. In this case, more than 2.6 trillion comparisons are made (one comparison is forward against forward, and one is forward against reverse; reverse against reverse is the same as forward against forward because of the inverse nucleotide arrangement of DNA). When searching human genes, the comparisons can be on the order of ''quadrillions'' (10^15) and take hours even on fast CPUs even with multi-core threading. Optimizing the comparison code saves time and power consumption, and is the focus of the profiling done below. | ||
| + | |||
| + | ==== Baseline comparison ==== | ||
| + | For these benchmarks, two different sequences of Epstein-Barr (EBV) and SARS-CoV-2 (Covid-19) virii will be used. The COVID-19 sequences, [https://www.ncbi.nlm.nih.gov/nuccore/OM002793.1 OM002793.1] and [https://www.ncbi.nlm.nih.gov/nuccore/OM003364.1 OM003364.1] are suitable for short duration benchmarks. The EBV sequences, [https://www.ncbi.nlm.nih.gov/nuccore/KC207814.1 KC207814.1] and [https://www.ncbi.nlm.nih.gov/nuccore/NC_007605.1 NC_007605.1] (canonical), are for longer benchmarks in which slight performance gains are measured. Each pair member in the two pairs of sequences are nearly identical, so when analysis of branch prediction for misses is needed, one from each pair can be compared against the other. | ||
| + | |||
| + | A typical comparison command looks like | ||
| + | |||
| + | <pre> | ||
| + | ./cmp_mp -l 12 -j 8 -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | </pre> | ||
| + | |||
| + | It is specifying a minimum length of 12 consecutive nucleotide matches and four forward slices and four reverse slices, with the two Covid-19 sequences. For benchmark clarity, the -s (silence) flag is set. The output from BE FreeBSD for only two threads: | ||
| + | |||
| + | <pre> | ||
| + | $ time ./cmp_mp -l 12 -j 1 -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | num args: 8 | ||
| + | -l optarg = 12 | ||
| + | Number of jobs must be even; adding +1 to n_jobs | ||
| + | input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta | ||
| + | o_st.st_size: 29766 , t_st.st_size: 29766 | ||
| + | min_pct_f = 1.00 | ||
| + | min_len = 12 | ||
| + | n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764 | ||
| + | number of sequences: 222 | ||
| + | longest sequence: 3270 ([19475, 19475), (22744, 22744)] | ||
| + | file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv | ||
| + | 5.80 real 11.19 user 0.00 sys | ||
| + | </pre> | ||
| + | |||
| + | This is the most accurate baseline comparison. With two threads, there are no slices. For absolute single-threaded execution, add -T to the flags. | ||
| + | |||
| + | To show the performance for additional threads, increase the number of jobs to the core count (4): | ||
| + | |||
| + | <pre> | ||
| + | $ time ./cmp_mp -l 12 -j 4 -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | num args: 8 | ||
| + | -l optarg = 12 | ||
| + | input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta | ||
| + | o_st.st_size: 29766 , t_st.st_size: 29766 | ||
| + | min_pct_f = 1.00 | ||
| + | min_len = 12 | ||
| + | n_jobs = 4, map_size = 14882 (0x3a22), slice_mod 0, against_sz 29764 | ||
| + | number of sequences: 223 | ||
| + | longest sequence: 3270 ([19475, 19475), (22744, 22744)] | ||
| + | file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_4_12_0_100_nuc.csv | ||
| + | 2.91 real 11.21 user 0.00 sys | ||
| + | </pre> | ||
| + | |||
| + | A change to -j 16, the maximum thread-core count, yields | ||
| + | |||
| + | <pre> | ||
| + | $ time ./cmp_mp -l 12 -j 16 -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | num args: 8 | ||
| + | -l optarg = 12 | ||
| + | input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta | ||
| + | o_st.st_size: 29766 , t_st.st_size: 29766 | ||
| + | min_pct_f = 1.00 | ||
| + | min_len = 12 | ||
| + | n_jobs = 16, map_size = 3720 (0xe88), slice_mod 4, against_sz 29764 | ||
| + | number of sequences: 226 | ||
| + | longest sequence: 2845 ([19475, 19475), (22319, 22319)] | ||
| + | file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_16_12_0_100_nuc.csv | ||
| + | 1.05 real 15.39 user 0.03 sys | ||
| + | </pre> | ||
| + | |||
| + | Notice the impact of slicing - there are more matching sequences, and the longest sequence is shorter. | ||
| + | |||
| + | There can be a minor performance benefit by double-loading the thread-core count: | ||
| + | |||
| + | <pre> | ||
| + | $ time ./cmp_mp -l 12 -j 32 -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | num args: 8 | ||
| + | -l optarg = 12 | ||
| + | input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta | ||
| + | o_st.st_size: 29766 , t_st.st_size: 29766 | ||
| + | min_pct_f = 1.00 | ||
| + | min_len = 12 | ||
| + | n_jobs = 32, map_size = 1860 (0x744), slice_mod 4, against_sz 29764 | ||
| + | thread[1] had no matches | ||
| + | number of sequences: 234 | ||
| + | longest sequence: 1860 ([13020, 13020), (14879, 14879)] | ||
| + | file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_32_12_0_100_nuc.csv | ||
| + | 1.02 real 15.60 user 0.02 sys | ||
| + | </pre> | ||
=== pmcstat === | === pmcstat === | ||
| + | |||
| + | Default setting is -w 5 for five second output with per interval incrementing. -C for cumulative counting. | ||
| + | |||
| + | <pre> | ||
| + | time pmcstat -w 1 -v -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | </pre> | ||
| + | |||
| + | No load w/ smt=1 | ||
| + | |||
| + | <pre> | ||
| + | $ time pmcstat -w 1 -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -T -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | num args: 9 | ||
| + | -l optarg = 12 | ||
| + | multithreading disabled | ||
| + | input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta | ||
| + | o_st.st_size: 29766 , t_st.st_size: 29766 | ||
| + | min_pct_f = 1.00 | ||
| + | min_len = 12 | ||
| + | n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764 | ||
| + | # p/pm_l1_icache_miss | ||
| + | 30312 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 1459 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 number of sequences: 222 | ||
| + | longest sequence: 3270 ([19475, 19475), (22744, 22744)] | ||
| + | file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv | ||
| + | |||
| + | 2399 | ||
| + | 11.18 real 0.01 user 0.00 sys | ||
| + | |||
| + | </pre> | ||
| + | |||
| + | No load w/ smt=4 | ||
| + | |||
| + | <pre> | ||
| + | $ time pmcstat -w 1 -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -T -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | num args: 9 | ||
| + | -l optarg = 12 | ||
| + | multithreading disabled | ||
| + | input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta | ||
| + | o_st.st_size: 29766 , t_st.st_size: 29766 | ||
| + | min_pct_f = 1.00 | ||
| + | min_len = 12 | ||
| + | n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764 | ||
| + | # p/pm_l1_icache_miss | ||
| + | 47223 | ||
| + | 0 | ||
| + | 90369 | ||
| + | 0 | ||
| + | 90800 | ||
| + | 64188 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 number of sequences: 222 | ||
| + | longest sequence: 3270 ([19475, 19475), (22744, 22744)] | ||
| + | file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv | ||
| + | |||
| + | 2411 | ||
| + | 11.18 real 0.01 user 0.00 sys | ||
| + | </pre> | ||
| + | |||
| + | Load w/ smt=1 | ||
| + | |||
| + | <pre> | ||
| + | $ time pmcstat -w 1 -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -T -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | num args: 9 | ||
| + | -l optarg = 12 | ||
| + | multithreading disabled | ||
| + | input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta | ||
| + | o_st.st_size: 29766 , t_st.st_size: 29766 | ||
| + | min_pct_f = 1.00 | ||
| + | min_len = 12 | ||
| + | n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764 | ||
| + | # p/pm_l1_icache_miss | ||
| + | 30449 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 1493 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 number of sequences: 222 | ||
| + | longest sequence: 3270 ([19475, 19475), (22744, 22744)] | ||
| + | file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv | ||
| + | |||
| + | 2569 | ||
| + | 11.17 real 0.00 user 0.00 sys | ||
| + | </pre> | ||
| + | |||
| + | Load w/ smt=4 | ||
| + | |||
| + | <pre> | ||
| + | $ time pmcstat -w 1 -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -T -s CVD_OM002793.1 CVD_OM003364.1 | ||
| + | num args: 9 | ||
| + | -l optarg = 12 | ||
| + | multithreading disabled | ||
| + | input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta | ||
| + | o_st.st_size: 29766 , t_st.st_size: 29766 | ||
| + | min_pct_f = 1.00 | ||
| + | min_len = 12 | ||
| + | n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764 | ||
| + | # p/pm_l1_icache_miss | ||
| + | 61484 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 2348 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 | ||
| + | 0 number of sequences: 222 | ||
| + | longest sequence: 3270 ([19475, 19475), (22744, 22744)] | ||
| + | file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv | ||
| + | |||
| + | 7596 | ||
| + | 19.84 real 0.00 user 0.01 sys | ||
| + | </pre> | ||
=== perf === | === perf === | ||
Latest revision as of 10:01, 16 July 2025
This article discusses profiling symmetric multithreading (SMT) on the POWER9 architecture. It uses both Big-endian FreeBSD with pmcstat and Debian with perf.
The knowledge presented here was derived from a variety of sources which can be found in the #Additional Resources section.
Contents
Symmetric multithreading (SMT)
SMT principles
SMT is "multi-thread" not "multi-core"
This is an important distinction. SMT is a technology that increases throughput of instructions through parallelization where there are under-used CPU components. While SMT4 can support four threads per core and SMT8 can support eight threads per core, this is not an additional three and seven cores, respectively. There are trade-offs and benefits. Per-thread performance declines with increasing utilization of SMT levels, but overall performance and power consumption efficiency increase. Note that IBM did not market SMT as "multi-core," while several media sites conflated SMT with increased core count.
Comparison to RISC-V HARTs
Benchmark code
Description of comparison code (cmp_mp)
The code being profiled and used for benchmarking is the genomic comparison code that the M. P. Janson Institute for Analytical Medicine uses to look for molecular mimicry between pathogens and human tissue and hormones. This code uses a variety of techniques to compare nucleotide and amino acid sequences. There are both byte-by-byte and vector versions. The raw source code can be found at TBD
Fundamentally the algorithm just compares characters in two sequences for matching. The goal is to match consecutive changing characters and record the string and location of the matches. Unlike strstr or similar functions, the string to be matched is not known in advance. The first character in one sequence is compared against the first character in the other sequence. If it matches, the second characters are compared. If they match, the third characters are compared, and so on, until the characters do not match. If the length of the matched string is below the minimum desired length, the match is discarded and the comparisons start over at the next character. This can "slide" the comparisons relative to each other, so if the first four characters match but not the fifth, the comparison starts over at the first character of one sequence and the fifth character of the other. If there is a match of sufficient length, that two-dimensional region is marked as an "exclusion region" and not searched again.
The code can run N number of user-specified pthreads in parallel (-j N), by slicing one of the two sequences into smaller pieces and stitching the results together. N must be an even number (one forward, one reverse). Some matches are lost at the boundaries between slices when one or both parts are less than the minimum length. The execution time is extremely sensitive to comparisons made; adding checks to determine if a match falls on a slice boundary adds significant overhead. For large sequences, the odds of a matching string falling on a boundary are very low; for small sequences, running fewer threads is effective without costing significant time delays. For absolute accuracy, run only two threads (one forward and one in reverse).
Description of data
Nucleotides are denoted as A, T, C, and G. There are three nucleotides in an amino acid, and 23 amino acids in humans, which leads to "isocodon" amino acids. These have the same function but different nucleotide combinations. There are amino acids that denote the initiation and termination of a gene. The amino acid is determined by its offset from the initiation amino acid (ATG, methionine), which starts an "Open Reading Frame (ORF)." ORFs can overlap. DNA has a forward ("sense") and backward ("anti-sense") direction, so there are three possible amino acids reading forward, and three when reading backwards. Nucleotide sequences are usually published in the forward direction, and the other strand's nucleotide matching the forward direction is inferred (A matches T, and C matches G, and vice versa). Genome sequences can be obtained from the NIH National Library of Medicine gene database, as well as in other places. The particular sequence is identified by its "accession number," and sequencing of the same organism by different labs does lead to different accession numbers. Some sequences are "canonical," meaning the generally accepted sequence that is often an assembly of accessions, and others are "contributions," meaning independently obtains sequences submitted for recording.
A typical "FASTA" file contains data as
TATATTAGAGTAGGAGCTAGAAAATCAGCACCTTTAATTGAATTGTGCGTGGATGAGGCTGGTTCTAAATCACCCATTCAGTACATCGATATCGGTAATTATACAGTT...
The most common comparison method is BLAST, which uses a database of pre-identified nucleotide sequences. This is useful for rapidly finding large matches, but does not offer granularity for exploring other aspects of the genome. The above method allows matching of nucleotide and amino acid sequences on both position one and position two nucleotide. This often reveals "repeat motifs" where a sequence of nucleotides is repeated multiple times within a pathogen. The current thinking is that this is an "immuno-evasive strategy," allowing the pathogen to escape detection by the host's immune system. Additionally, by recording the location of the matches, the distance between matches can be calculated. This is important in deciding if a host antibody would identify healthy tissue and hormones as pathogenic, by having similar shapes. The above algorithm also allows for detecting "SIDs," which are "Substitutions, Insertions, and Deletions," which occur regularly in rapidly evolving pathogens such as viruses.
Sample output
The output is a csv file that is run through some custom image generation code using libjpeg. An example of the processed data is below
The image shows matching sequences between two bacteria, B. burgdorferi, which causes Lyme disease, and B. miyamotoi, a bacteria in the same Borrelia family. Matches are occurring in both the forward and reverse directions. In this particular case, the B. miyamotoi sequence was assembled from fragments in both the "sense" and "antisense" direction. This is an artifact of how PCR is done, but has to be considered when doing analysis. Running only forward matching can lead to missed matches (a "false negative"). This comparison is particularly important because some people will exhibit symptoms of Lyme disease but only test weakly positive or not at all for Lyme disease. This is known in science but not practiced in medicine, leading to erroneous conclusions about the cause of the patient's symptoms (see Borrelia miyamotoi infection leads to cross-reactive antibodies to the C6 peptide in mice and men).
The B. burgdefori sequence is 1,455,375 nucleotides long, and the B. miyamotoi sequence is 907,293 nucleotides long. Each nucleotide is compared at least once, and when there are partial matches too short to place the nucleotide into an exclusion region, it may be compared more than once. In this case, more than 2.6 trillion comparisons are made (one comparison is forward against forward, and one is forward against reverse; reverse against reverse is the same as forward against forward because of the inverse nucleotide arrangement of DNA). When searching human genes, the comparisons can be on the order of quadrillions (10^15) and take hours even on fast CPUs even with multi-core threading. Optimizing the comparison code saves time and power consumption, and is the focus of the profiling done below.
Baseline comparison
For these benchmarks, two different sequences of Epstein-Barr (EBV) and SARS-CoV-2 (Covid-19) virii will be used. The COVID-19 sequences, OM002793.1 and OM003364.1 are suitable for short duration benchmarks. The EBV sequences, KC207814.1 and NC_007605.1 (canonical), are for longer benchmarks in which slight performance gains are measured. Each pair member in the two pairs of sequences are nearly identical, so when analysis of branch prediction for misses is needed, one from each pair can be compared against the other.
A typical comparison command looks like
./cmp_mp -l 12 -j 8 -s CVD_OM002793.1 CVD_OM003364.1
It is specifying a minimum length of 12 consecutive nucleotide matches and four forward slices and four reverse slices, with the two Covid-19 sequences. For benchmark clarity, the -s (silence) flag is set. The output from BE FreeBSD for only two threads:
$ time ./cmp_mp -l 12 -j 1 -s CVD_OM002793.1 CVD_OM003364.1
num args: 8
-l optarg = 12
Number of jobs must be even; adding +1 to n_jobs
input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta
o_st.st_size: 29766 , t_st.st_size: 29766
min_pct_f = 1.00
min_len = 12
n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764
number of sequences: 222
longest sequence: 3270 ([19475, 19475), (22744, 22744)]
file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv
5.80 real 11.19 user 0.00 sys
This is the most accurate baseline comparison. With two threads, there are no slices. For absolute single-threaded execution, add -T to the flags.
To show the performance for additional threads, increase the number of jobs to the core count (4):
$ time ./cmp_mp -l 12 -j 4 -s CVD_OM002793.1 CVD_OM003364.1
num args: 8
-l optarg = 12
input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta
o_st.st_size: 29766 , t_st.st_size: 29766
min_pct_f = 1.00
min_len = 12
n_jobs = 4, map_size = 14882 (0x3a22), slice_mod 0, against_sz 29764
number of sequences: 223
longest sequence: 3270 ([19475, 19475), (22744, 22744)]
file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_4_12_0_100_nuc.csv
2.91 real 11.21 user 0.00 sys
A change to -j 16, the maximum thread-core count, yields
$ time ./cmp_mp -l 12 -j 16 -s CVD_OM002793.1 CVD_OM003364.1
num args: 8
-l optarg = 12
input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta
o_st.st_size: 29766 , t_st.st_size: 29766
min_pct_f = 1.00
min_len = 12
n_jobs = 16, map_size = 3720 (0xe88), slice_mod 4, against_sz 29764
number of sequences: 226
longest sequence: 2845 ([19475, 19475), (22319, 22319)]
file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_16_12_0_100_nuc.csv
1.05 real 15.39 user 0.03 sys
Notice the impact of slicing - there are more matching sequences, and the longest sequence is shorter.
There can be a minor performance benefit by double-loading the thread-core count:
$ time ./cmp_mp -l 12 -j 32 -s CVD_OM002793.1 CVD_OM003364.1
num args: 8
-l optarg = 12
input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta
o_st.st_size: 29766 , t_st.st_size: 29766
min_pct_f = 1.00
min_len = 12
n_jobs = 32, map_size = 1860 (0x744), slice_mod 4, against_sz 29764
thread[1] had no matches
number of sequences: 234
longest sequence: 1860 ([13020, 13020), (14879, 14879)]
file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_32_12_0_100_nuc.csv
1.02 real 15.60 user 0.02 sys
pmcstat
Default setting is -w 5 for five second output with per interval incrementing. -C for cumulative counting.
time pmcstat -w 1 -v -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -s CVD_OM002793.1 CVD_OM003364.1
No load w/ smt=1
$ time pmcstat -w 1 -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -T -s CVD_OM002793.1 CVD_OM003364.1
num args: 9
-l optarg = 12
multithreading disabled
input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta
o_st.st_size: 29766 , t_st.st_size: 29766
min_pct_f = 1.00
min_len = 12
n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764
# p/pm_l1_icache_miss
30312
0
0
0
0
1459
0
0
0
0
0 number of sequences: 222
longest sequence: 3270 ([19475, 19475), (22744, 22744)]
file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv
2399
11.18 real 0.01 user 0.00 sys
No load w/ smt=4
$ time pmcstat -w 1 -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -T -s CVD_OM002793.1 CVD_OM003364.1
num args: 9
-l optarg = 12
multithreading disabled
input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta
o_st.st_size: 29766 , t_st.st_size: 29766
min_pct_f = 1.00
min_len = 12
n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764
# p/pm_l1_icache_miss
47223
0
90369
0
90800
64188
0
0
0
0
0 number of sequences: 222
longest sequence: 3270 ([19475, 19475), (22744, 22744)]
file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv
2411
11.18 real 0.01 user 0.00 sys
Load w/ smt=1
$ time pmcstat -w 1 -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -T -s CVD_OM002793.1 CVD_OM003364.1
num args: 9
-l optarg = 12
multithreading disabled
input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta
o_st.st_size: 29766 , t_st.st_size: 29766
min_pct_f = 1.00
min_len = 12
n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764
# p/pm_l1_icache_miss
30449
0
0
0
0
1493
0
0
0
0
0 number of sequences: 222
longest sequence: 3270 ([19475, 19475), (22744, 22744)]
file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv
2569
11.17 real 0.00 user 0.00 sys
Load w/ smt=4
$ time pmcstat -w 1 -p pm_l1_icache_miss ./cmp_mp -l 12 -j 2 -T -s CVD_OM002793.1 CVD_OM003364.1
num args: 9
-l optarg = 12
multithreading disabled
input/CVD_OM002793.1.fasta, input/CVD_OM003364.1.fasta
o_st.st_size: 29766 , t_st.st_size: 29766
min_pct_f = 1.00
min_len = 12
n_jobs = 2, map_size = 29764 (0x7444), slice_mod 0, against_sz 29764
# p/pm_l1_icache_miss
61484
0
0
0
0
0
0
0
0
2348
0
0
0
0
0
0
0
0
0 number of sequences: 222
longest sequence: 3270 ([19475, 19475), (22744, 22744)]
file name: mp_CVD_OM003364.1_vs_CVD_OM002793.1_2_12_0_100_nuc.csv
7596
19.84 real 0.00 user 0.01 sys
perf
Additional Resources
POWER9 Performance Monitoring Unit User Guide v12
POWER CPU Memory Affinity 3 - Scheduling processes to SMT and Virtual Processors